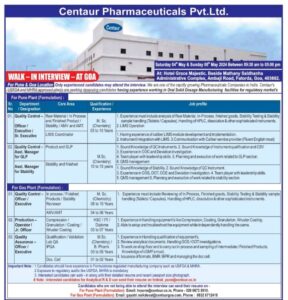
WALK-IN INTERVIEWS CENTAUR PHARMACEUTICALS FOR QA/QC/PRODUCTION
Centaur Pharmaceuticals, commenced pharmaceutical manufacturing operations in Mumbai and has now evolved into a vertically integrated pharmaceutical company. Centaur House, our corporate headquarters, is located in Mumbai, the commercial capital of India. Centaur has a strong matrix of capabilities across the pharmaceutical value chain including API, formulations, Contract Research and Manufacturing Services, Clinical Research, R&D, regulatory and marketing.
JOB DESCRIPTION
Walk – in interview Details
Interview Date – 04 May 2024
Time – 09:30 AM to 02:00 PM
JOB Work Location – Maharashtra
DEPARTMENT – Quality Control (Formulation)
Qualification – M. Sc. Chemistry
Experience – 05 – 10 Years
Work Location – Pune Plant
DEPARTMENT – Quality Control (LIMS Coordinator)
Qualification – M. Sc. Chemistry
Experience – 08 – 10 Years
Work Location – Pune Plant
JOB Work Location – Goa
DEPARTMENT – Quality Control (LIMS Coordinator)
Qualification – M. Sc. Chemistry
Experience – 10 – 15 Years
Work Location – Goa Plant
DEPARTMENT – Quality Assurance
Qualification – M.Sc. Chemistry/ B. Pharm
Experience – 03 – 05 Years
Work Location – Goa Plant
DEPARTMENT – Production ( Operator )
Qualification – HSC/ITI/ Diploma
Experience – 03 – 07 Years
Work Location – Goa Plant
Important Note:
- Interested candidates can walk-in along with their detailed resume and recent passport size photograph.
- Candidates who are not able to attend the interview can send their resume to the provided email addresses and contact numbers.
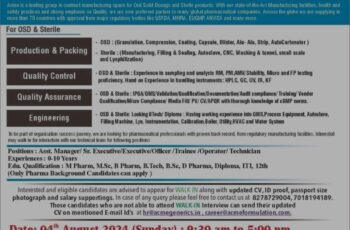
Walk – in interview Details Interview
Date – 04 & 05 May 2024
Time – 09:30 AM to 05:00 PM
Venue – Hotel Grace Majestic, Beside Mathany Saidhanha Administrative Complex, Ambaji Road, Fatorda, Goa, 403602.
Contact
For Pune Plant (Formulation) :
Email: hrpune@centaur.co.in,
Phone 020 6673 9510.
For Goa Plant (Formulation) :
Email: gayatri.naikdesai@centaurgoa.com,
Phone: 0832 6712410
Please Note : Candidates should have experience in Formulations regulated manufacturing company such as USFDA & MHRA. Exposure to regulatory audits like USFDA, MHRA is mandatory.