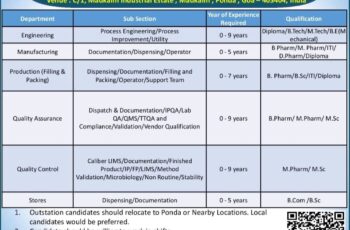
Centaur Pharmaceuticals Pvt Ltd., Pune
Centaur Pharmaceutical is the largest specialty drug manufacturing organization, providing high-quality, affordable medicines trusted by healthcare professionals, including USFDA and MHRA. We are one of the rapidly growing pharmaceutical companies in India. To keep pace with our growth plan, we require young and energetic professionals for our Pune site, with experience in Formulation/Oral Solid Dosage.
JOB DESCRIPTION
DEPARTMENT – Quality Assurance
Designation – Officer/Executive/Sr. Executive
Experience – 04 to 08 years
Qualification – B. Pharm./M. Pharm.
Core Area – QMS/Validation/Investigation/APQR/Stability Study/Auditing/Compliances/Training/IPQA
Job Profile:
- Initiation, review, and tracking of QMS elements such as deviation, change control, CAPA, market complaints.
- Assure compliance with regulatory requirements.
- Ensure manufacturing activities are carried out as per the approved procedure and comply with cGMP.
- Preparation, review, and implementation of QA SOP, and evaluation and implementation of CQA SOP.
- Prepare and review APQR.
DEPARTMENT – Quality Assurance
Designation – Quality Assurance – Quality Management System
Experience – 12 to 14 years
Qualification – B. Pharm./M. Pharm.
Core Area – QMS/Validation/Investigation/APQR/Stability Study/Auditing/Compliances/Training/IPQA
Job Profile:
- Operate core QA processes like deviation, non-conformance, change control, document review, auditing, and training as instructed by QA Management.
- Review analytical data and ensure the team has no overdue training or site actions.
- Conduct internal and external audits to assess QMS effectiveness and identify improvement areas.
DEPARTMENT – Analytical Quality Assurance Functions
Experience – 12 to 14 years
Qualification – B. Pharm./M. Pharm.
Core Area – Analytical QA Functions
Job Profile:
- Oversee the review and approval of manufacturing batch records, analytical data, and other documentation to ensure product quality and regulatory compliance.
- Ensure adherence to GMP, GLP, and QMS guidelines in all analytical activities.
- Collaborate with cross-functional teams to resolve quality issues and implement corrective actions.
JOB Details
Contact – hrpune@centaur.co.in